Protocols for use of SuperNova v428 conjugated antibodies in a variety of flow cytometry applications
Geraldine Biechele, Senior Technician Development Science | Beckman Coulter Life Sciences
Xavier Pineau, Senior Technician Development Science | Beckman Coulter Life Sciences
Fabien Jusdado Fernandez, Technician Development Science | Beckman Coulter Life Sciences
Anne-Claire Giai, Technician Development Science | Beckman Coulter Life Sciences
Brice Ezzouaouy, Senior Product Manager | Beckman Coulter Life Sciences
Introduction
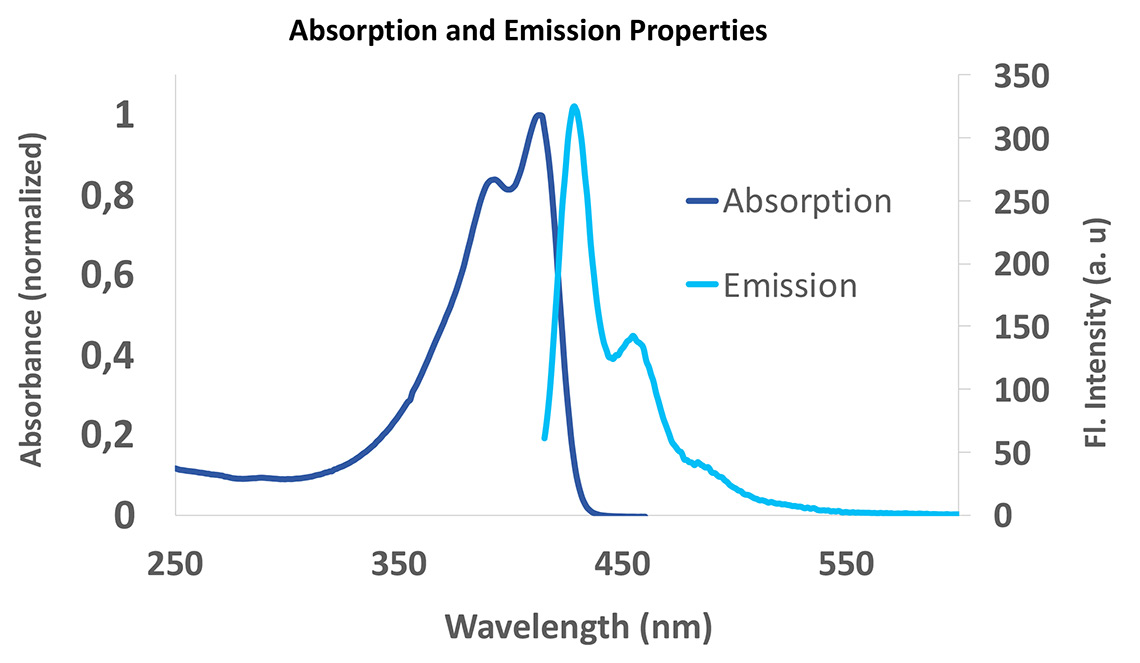
SuperNova dyes are a new family of polymer dyes for flow cytometry applications developed by Beckman Coulter Life Sciences. SuperNova v428 (SN v428) is the first dye of the series, optimally excited by the violet laser (405 nm) with an emission peak at 428 nm.
Figure 1. Absorption and emission spectra (λexc = 405 nm of SuperNova v428 in PBS).
Conjugated to antibodies, SuperNova v428 not only delivers unique brightness but also improves the overall staining index thanks to a proprietary formulation minimizing non-specific staining. SuperNova conjugated antibodies are optimal for the analysis of dimly expressed markers, providing greater confidence in results. They constitute a brighter alternative to fluorochromes such as Pacific Blue, and less background compared to Brilliant Violet 421 conjugated antibodies (BV 421).
Flow cytometry is used for a variety of applications and conjugated antibodies are the cornerstone of most assays. They must be compatible with a wide range of sample types and sample preparation protocols. Depending on the experiments, minor adjustments to the protocols can lead to significant improvement of the results obtained. This application note shares some of the results obtained by our Research and Development teams during the development of SuperNova conjugated antibodies. It shows comparison to other widely used fluorochromes, and provides tips and tricks for successfully planning multicolor flow cytometry experiments using SuperNova conjugated antibodies for these conditions and protocols:
- Staining index comparison with other fluorochromes
- Spillover comparison with other fluorochromes
- Comparison of several lysing solutions, with and without wash
- Impact of formaldehyde fixation
- Use in multicolor experiments
- Staining of PBMCs
Method & Results
The staining index measures the separation between negative and positive cell populations using a given conjugated antibody. It takes in consideration both the relative brightness on a specific flow cytometer, and the non-specific staining or background, calculated as the difference between the positive and negative Mean Fluorescence Intensity (MFI) divided by two times the standard deviation of the negative population. Conjugated antibodies with higher staining index are preferred for flow cytometry applications, as they are bright enough to enable identification of dimly expressed cell populations and provide greater confidence in results thanks to low non-specific staining.
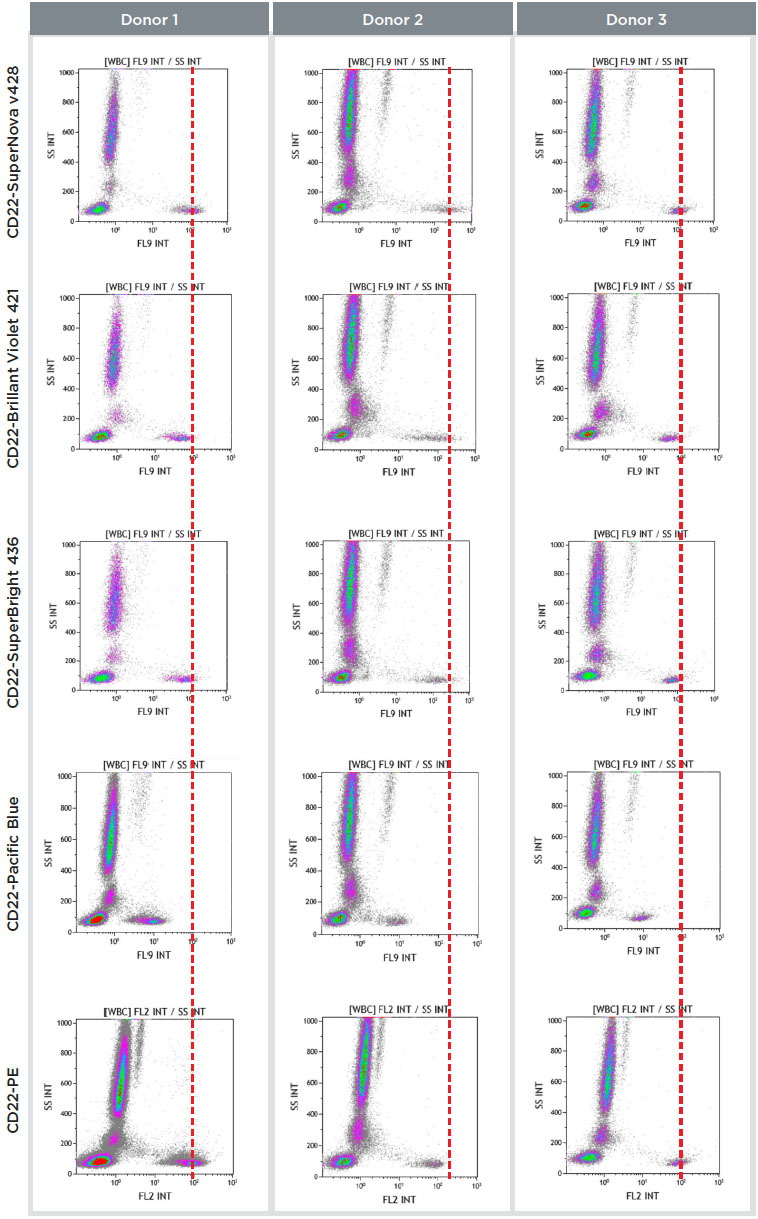
Figure 2. Staining patterns of 3 normal whole blood samples stained with CD22 conjugated to SNv428, Brilliant Violet 421, SuperBright 436, Pacific Blue and PE.
The SuperNova v428 staining index was compared to various fluorochromes: PE, one of the most commonly used dyes in flow cytometry, and three violet laser excitable dyes with similar emission properties: Pacific Blue, Brilliant Violet 421 (BV421) and Super Bright 436. The comparison was done staining normal whole blood samples (100μL: 5x105 cells/test) from 3 donors using CD22 antibodies conjugated to the different fluorochromes.
Results obtained are summarized in Figure 2 and Table 1.
Table 1. Lymphocytes and Monocytes MFI on 3 normal whole blood samples stained with CD22 conjugated to SNv428, Brilliant Violet 421, SuperBright 436, Pacific Blue and PE. N/A: Not Applicable
| MFI Analysis on Lymphocytes | Background Staining on Monocytes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD22 conjugated antibody | LY MFI- | LY MFI+ | Ratio RMFI % Test CD22 other Dye vs CD22-SNv428 | X Stdev LY | Staining Index | Ratio Staining Index ref SNv428 % | MO MFI- | Ratio MFI % Test vs SNv428 | ||
| Donor 1 | CD22-SNv428 | 0,370 | 103,512 | N/A | 0,125 | 412,568 | N/A | 0,958 | N/A | |
| CD22-BV421 | 0,384 | 47,404 | 44% | 0,130 | 180,846 | 44% | 1,303 | 136% | ||
| CD22-SB436 | 0,419 | 66,355 | 57% | 0,159 | 207,346 | 50% | 1,107 | 116% | ||
| CD22-Pacific Blue | 0,356 | 8,685 | 9% | 0,104 | 40,043 | 10% | 0,945 | 99% | ||
| CD22-PE | 0,416 | 79,803 | 69% | 0,168 | 236,271 | 57% | 1,119 | 117% | ||
| Donor 2 | CD22-SNv428 | 0,316 | 216,221 | N/A | 0,120 | 899,604 | N/A | 0,685 | N/A | |
| CD22-BV421 | 0,331 | 83,604 | 37% | 0,127 | 327,846 | 36% | 0,946 | 138% | ||
| CD22-SB436 | 0,344 | 107,685 | 46% | 0,127 | 422,602 | 47% | 0,664 | 97% | ||
| CD22-Pacific Blue | 0,299 | 6,511 | 3% | 0,101 | 30,752 | 3% | 0,643 | 94% | ||
| CD22-PE | 0,378 | 69,641 | 27% | 0,155 | 223,429 | 25% | 1,029 | 150% | ||
| Donor 3 | CD22-SNv428 | 0,324 | 107,276 | N/A | 0,118 | 453,186 | N/A | 0,674 | N/A | |
| CD22-BV421 | 0,358 | 47,679 | 40% | 0,124 | 190,810 | 42% | 1,061 | 157% | ||
| CD22-SB436 | 0,382 | 74,757 | 59% | 0,144 | 258,247 | 57% | 0,784 | 116% | ||
| CD22-Pacific Blue | 0,329 | 8,686 | 8% | 0,104 | 40,178 | 9% | 0,659 | 98% | ||
| CD22-PE | 0,415 | 88,980 | 65% | 0,160 | 276,766 | 61% | 1,000 | 148% | ||
As shown in Figure 2 and Table 1, CD22-SNv428 conjugate presented greater brightness and staining index compared to CD22 conjugated to other dyes. Both brightness and non-specific staining are improved, making SuperNova v428 an ideal dye for the assessment of dimmest cell populations with greater confidence.
Tips and tricks for a successful experiment:
- SuperNova v428 is the brightest fluorochrome for its channel.
- SuperNova v428 conjugated antibodies are optimal for the assessment of dimly expressed cell populations.
- SuperNova v428 conjugated antibodies generate significantly less nonspecific staining, providing greater confidence in results.
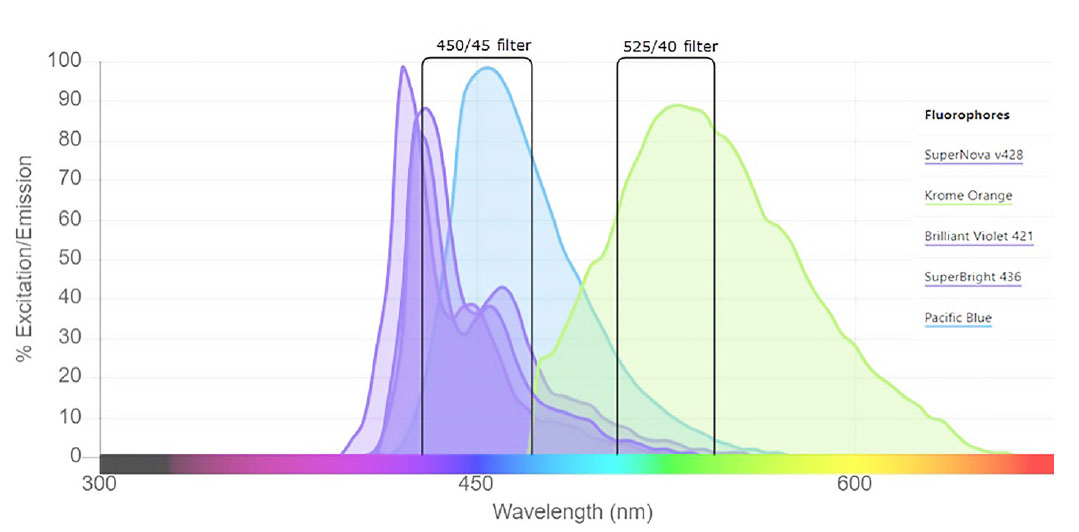
As flow cytometry panels tend to combine more and more fluorochromes to allow simultaneous assessment of more cell markers, compensation requirements due to spectral overlap between fluorochromes is a major aspect to consider when designing panels. SuperNova v428 only shows non-negligible spillover into the FL10 channel (e.g. 525/40 bandpass filter, commonly used for Krome Orange). As shown in figure 3, the emission pattern of SN v428 is similar to BV421 or SuperBright 436.
Figure 3. Emission properties of SN v428, BV421, SB436 and Pacific Blue, illustrating spillover into bandpass filter used for detection of Krome Orange.
SuperNova v428 is not cross-excited by other lasers of the flow cytometer beyond 450nm (see Figure 1).
The spillover from SuperNova v428 into FL10 was compared with Pacific Blue, BV421 and Super Bright 436 on 3 normal whole blood samples (100μL: 5x105 cells/test).
Table 2. Spillover for 3 normal whole blood samples stained with CD22 conjugated to SNv428, Brilliant Violet 421, SuperBright 436, Pacific Blue and PE.
| Spillover | ||
|---|---|---|
| CD22 | % FL9 in FL10 | |
| Donor 1 | CD22-SNv428 | 3,6 |
| CD22-BV421 | 2,5 | |
| CD22-SB436 | 3,4 | |
| CD22-Pacific Blue | 6,5 | |
| CD22-PE | N/A | |
| Donor 2 | CD22-SNv428 | 3,6 |
| CD22-BV421 | 2,4 | |
| CD22-SB436 | 3,4 | |
| CD22-Pacific Blue | 6,6 | |
| CD22-PE | N/A | |
| Donor 3 | CD22-SNv428 | 3,5 |
| CD22-BV421 | 2,4 | |
| CD22-SB436 | 3,3 | |
| CD22-Pacific Blue | 5,9 | |
| CD22-PE | N/A | |
Spillover from SuperNova v428 and associated compensation requirements are in the same order of magnitude as BV421 and SB436.
Tips and tricks for a successful experiment:
- SuperNova v428 generate similar spillover as BV421.
- SuperNova v428 is not cross excited by the red or blue laser of the flow cytometer.
- Compensation is required only in the FL10 channel. As the FL10 channel is commonly used for gating marker (Krome Orange), this spillover does not raise significant challenges.
The following lysing solutions were compared using a CD22-SuperNova v428:
- VersaLyse, an enzyme-based erythrocyte lysis commonly used for extracellular staining, with low stringency and usually gentle to cells. Protocols with fixation and with/without washing steps were compared
- OptiLyse C, a formaldehyde-glycerol based erythrocyte lysis designed for no-wash experiments primarily on Beckman Coulter instruments
- IOTest3 Lysing solution, an ammonium Chloride (NH4CL) based erythrocyte lysis, with a washing step
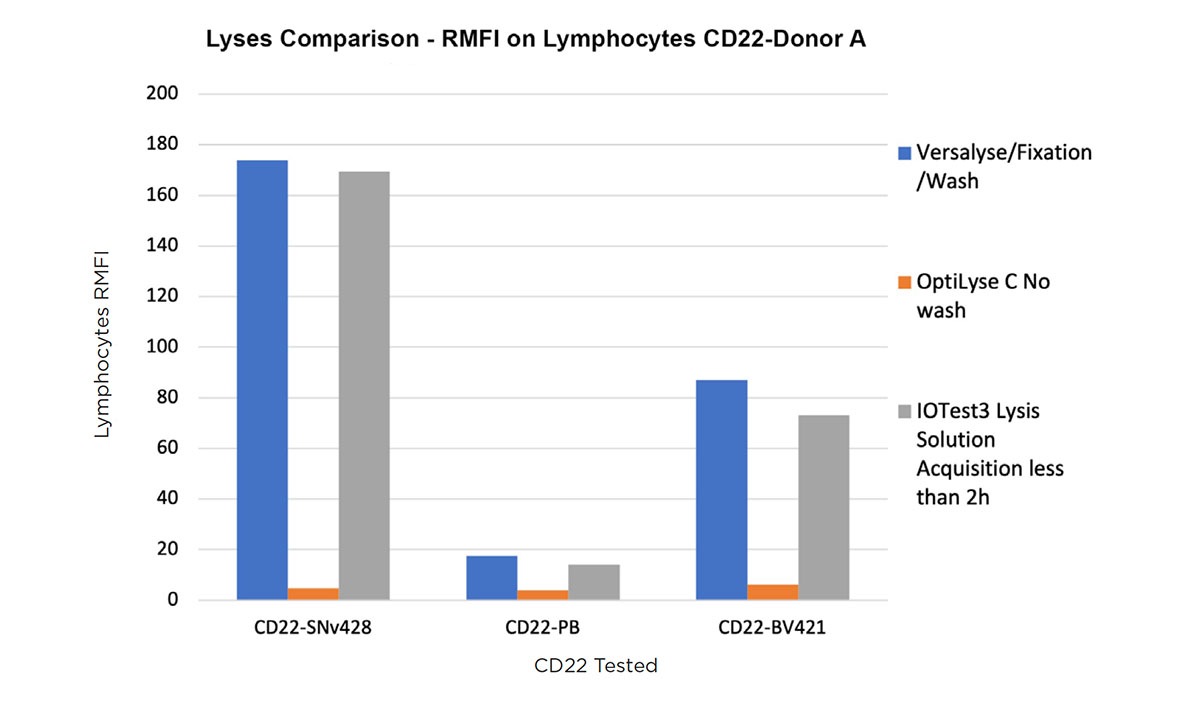
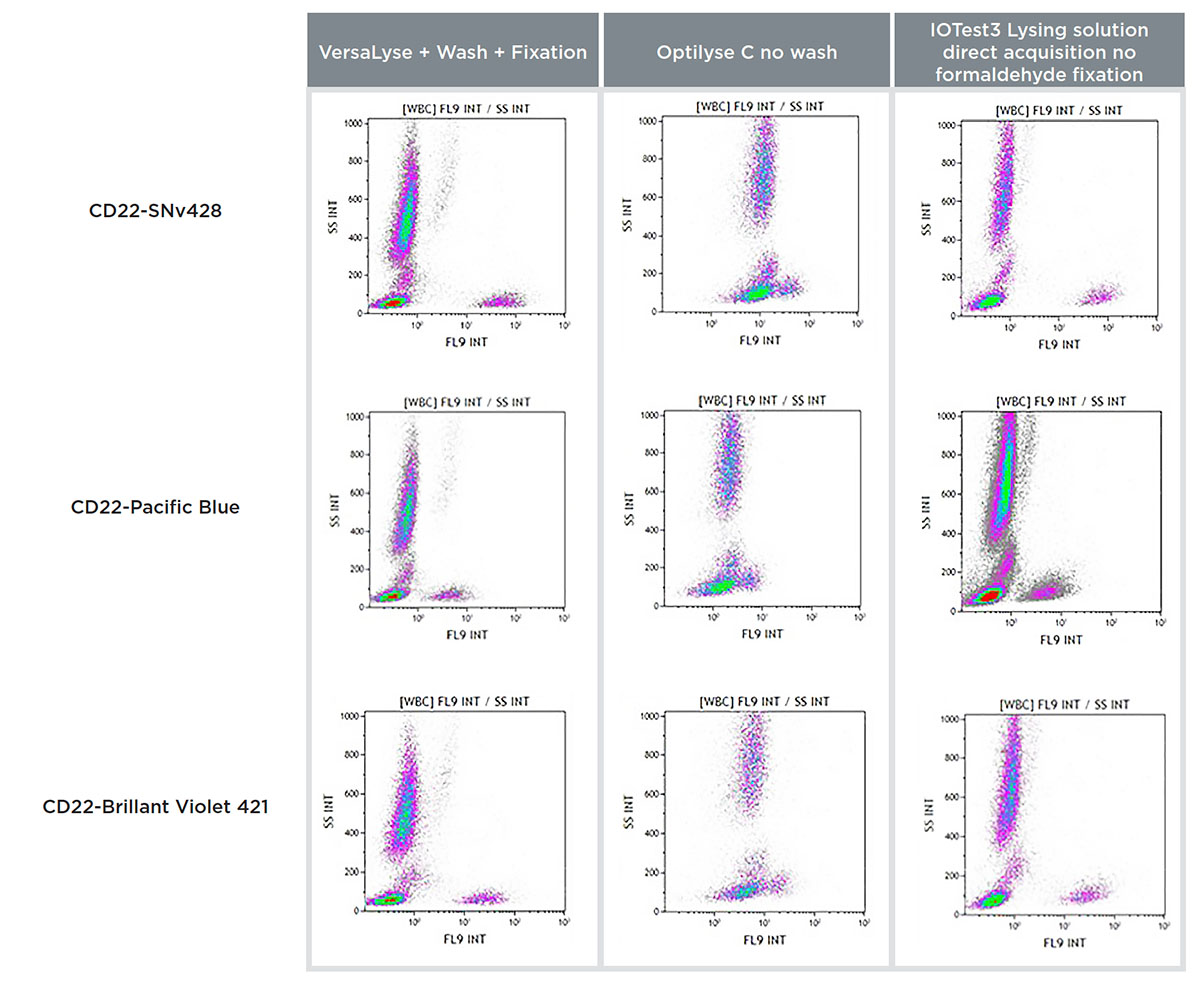
Figure 4 shows the RMFI (signal-to-noise ratio) obtained on a normal whole blood sample (100μL: 5x105 cells/test) lysed with the three lysing solutions for a CD22 conjugated to SuperNova v428, Pacific Blue and BV421.
Figure 4. Comparison of a CD22-SuperNova v428, Pacific Blue and BV421 staining on a normal whole blood sample with VersaLyse + Wash, IOTest3 Lysing Solution + wash and OptiLyse C no wash, showing RMFI values (top) and flow cytometry staining data (bottom).
Both VersaLyse and IOTest3 Lysing solutions delivered an optimal RMFI for the CD22-SNv428 conjugate, significantly superior to Pacific Blue and BV421. RMFI was poor in comparison with OptiLyse C no wash, for all 3 conjugates tested.
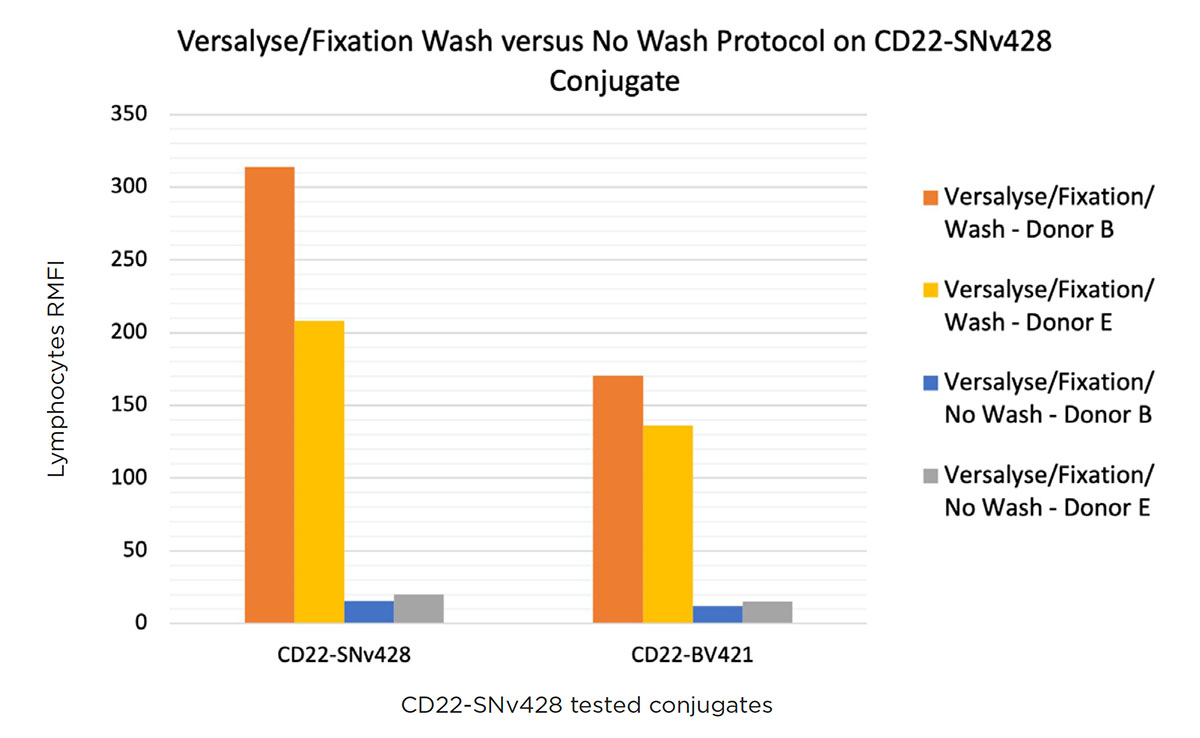
To evaluate if the poor discrimination obtained with OptiLyse C no wash is due to the absence of washing steps or the lysing solution itself, the VersaLyse + fixative protocol, which gave great results with a washing step, was tested without washing, on two donor (100μL: 5x105 cells/test normal whole blood samples).
As shown in Figure 5, the RMFI was about 10 times lower without wash compared to the protocol including washing steps. This significantly poorer separation between positive and negative cells in the context of a no-wash protocol was observed not only with SuperNova v428 but also with BV421.
Figure 5. RMFI comparison of a CD22-SuperNova v428, Pacific Blue and BV421 staining on two normal whole blood donor samples with VersaLyse + fixative no wash.
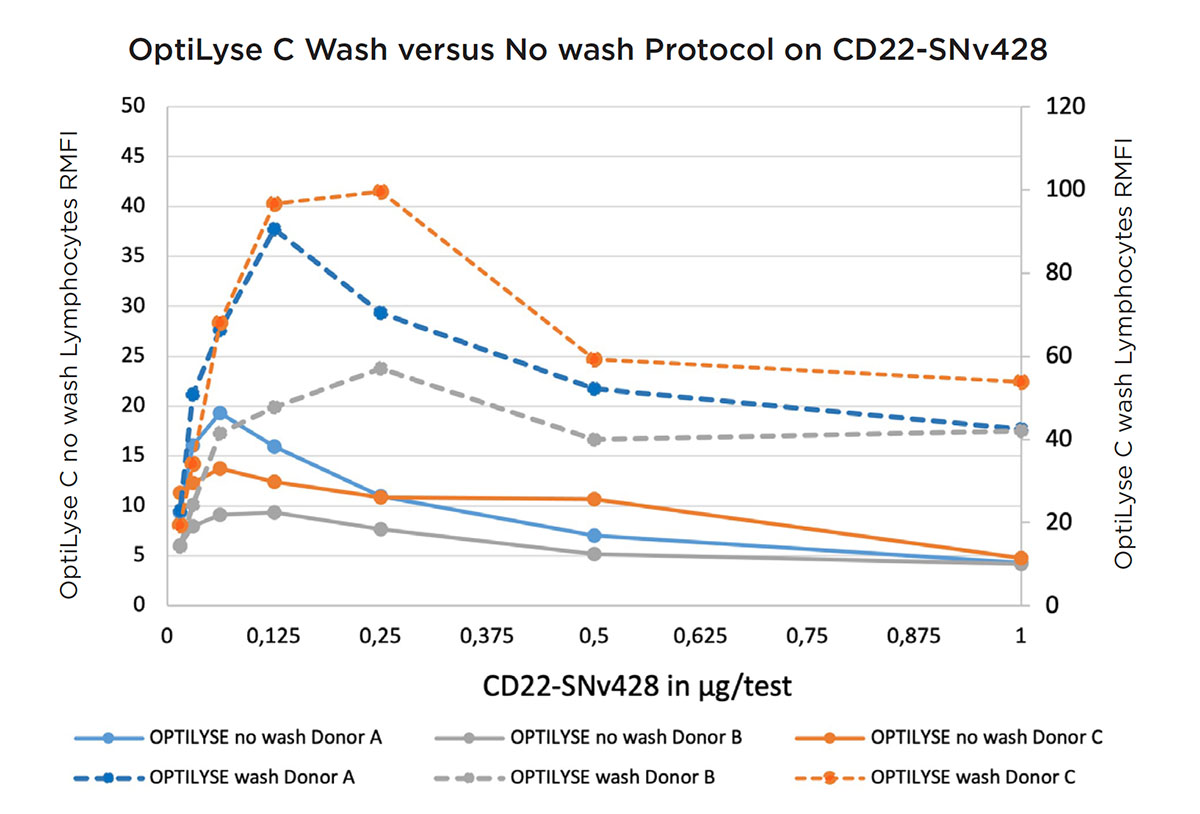
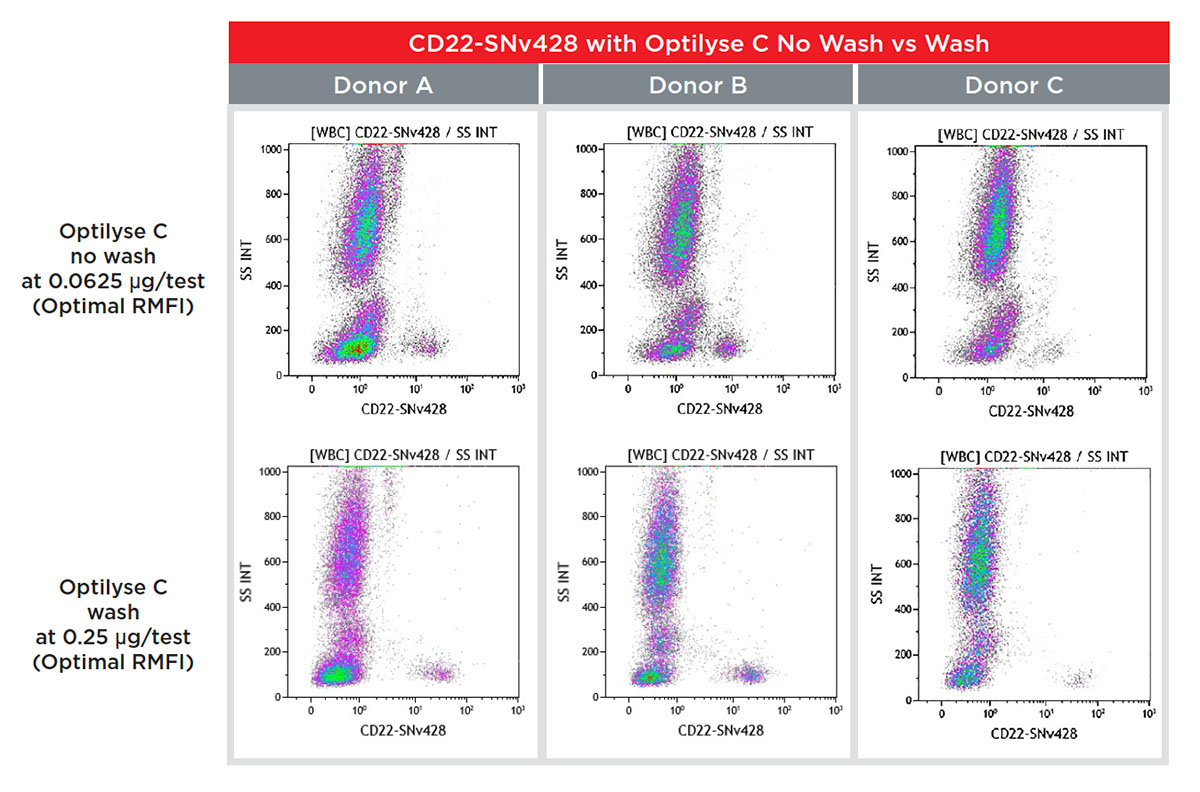
To improve the results and reduce the background, the CD22-SuperNova v428 conjugated Ab was titrated with OptiLyse C no wash protocol and wash protocol. A washing step is an optional possibility according to the Instruction For Use of OptiLyse C .
As shown in Figures 6 and 7, the signal-to-noise ratio for OptiLyse C wash was highly improved using the wash step, optimal RMFI was obtained at 0.25 μg/test.
Figure 6. Titration curve of CD22-SuperNova v428 on 3 donors, using OptiLyse C no wash (RMFI values shown on left axis) and wash (RMFI values shown on right axis).
Figure 7. Flow cytometry staining data of CD22-SuperNova v428 on 3 donor samples, using OptiLyse C no wash and wash procedures.
Tips and tricks for a successful experiment:
- VersaLyse + fixative and IOTest3 lysing solutions that include washing steps deliver optimal results.
- Signal-to-noise ratio is poor in no-wash conditions, both with SuperNova v428 and Pacific Blue/BV421.
- If a no-wash protocol is required, titrating down the conjugated antibody might help to reduce the background, though the staining index will remain significantly lower compared to protocols including a washing step.
- The addition of a washing step to the OptiLyse C protocol with a titration produces an RMFI closer to VersaLyse and IOTest3 Lysing solutions.
Sample fixation can be useful in a variety of cases, for instance in the context of intracellular staining (required to maintain integrity of surface markers prior to permeabilization, when assessed simultaneously with intracellular markers), or simply to provide flexibility when acquiring the sample on a flow cytometer at a later time.
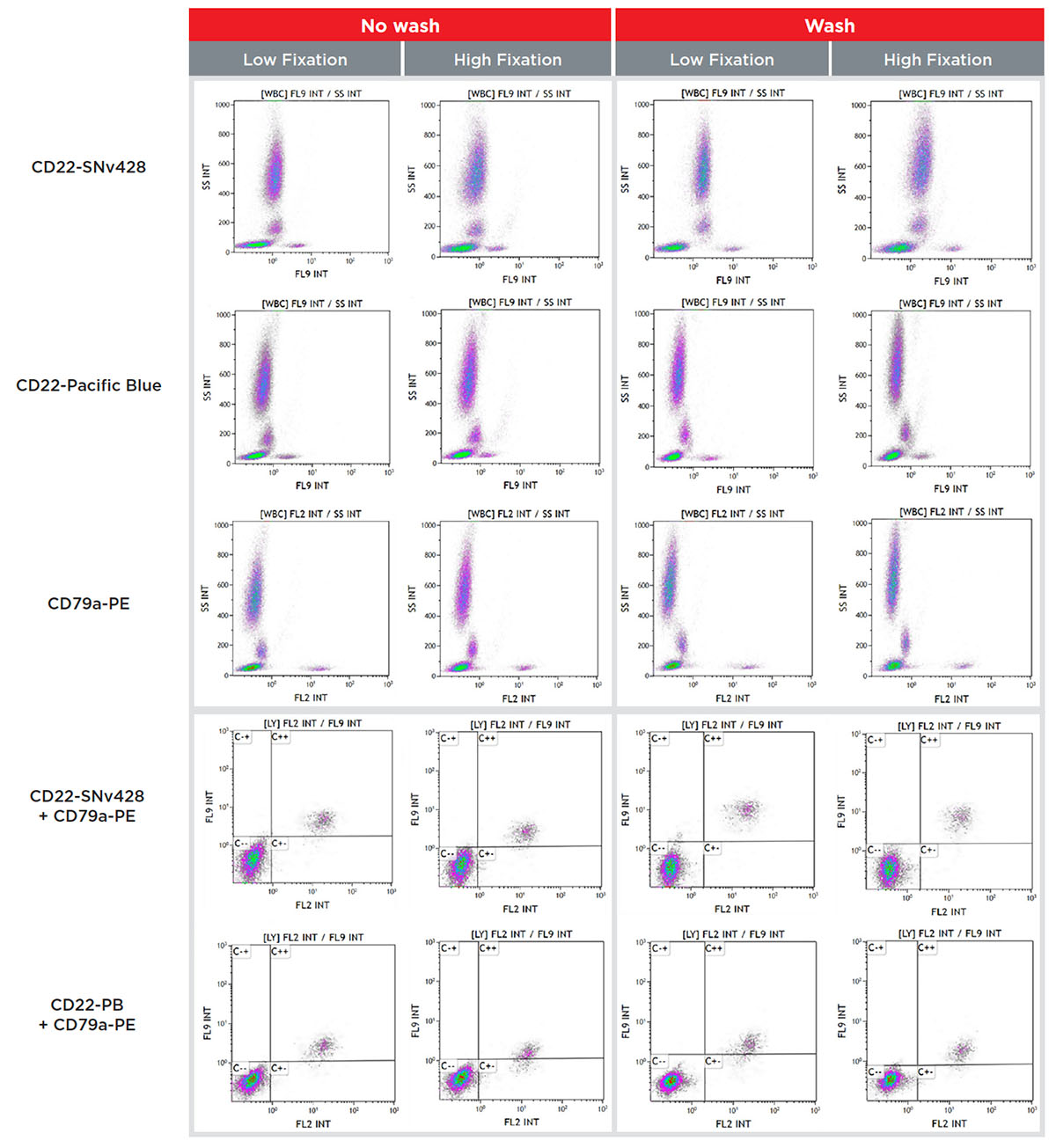
To assess the compatibility of SuperNova conjugated antibodies with intracellular staining protocols requiring formaldehyde-fixation, a CD22-SuperNova v428 conjugate was used to stain a sample of normal whole blood (50μL: 2.5x105 cells/test) prepared with different protocols from the PerFix-nc kit. PerFix-nc was developed to simplify the workload necessary for the intracellular staining sample preparation. Surface markers can be added together with intracellular markers and incubated simultaneously during the permeabilization step, after fixation. Four protocols are recommended in the instruction for use: low and high fixation, in no-wash and wash conditions.
As shown in Figure 8, the staining patterns of the CD22-SNv428 are similar between the low and high fixation protocols. Adding a washing step obviously improves the staining index though discrimination between negative and positive populations can also be done with the no-wash protocol, which provides significant workflow advantages.
Figure 8. Flow cytometry staining data of CD22-SuperNova v428 or Pacific Blue with CD79a-PE (intracellular marker) on a normal whole blood sample prepared with the four PerFix-nc protocols.
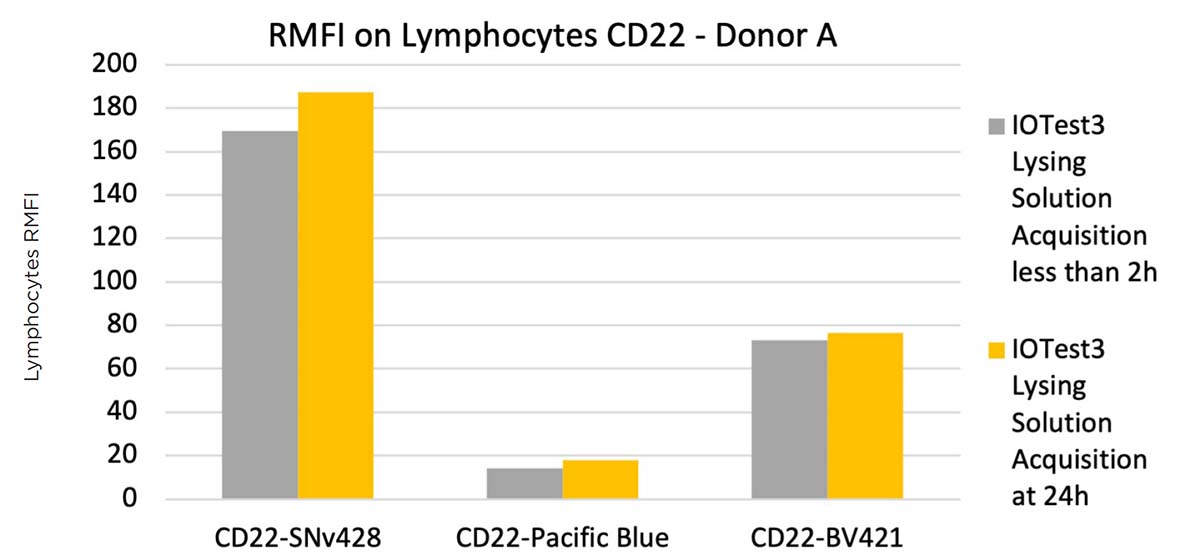
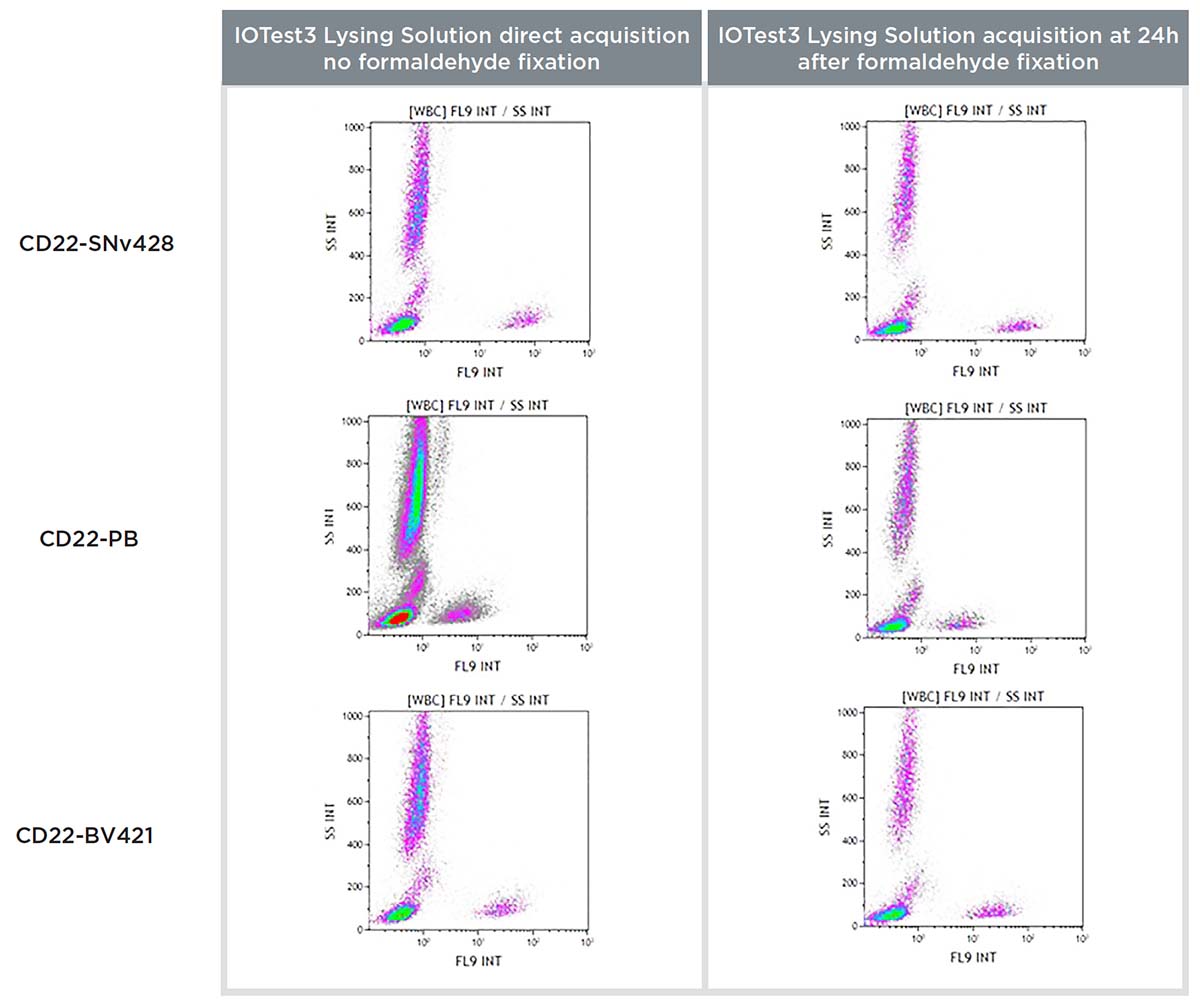
Formaldehyde can further impact some fluorochromes when fixation is done after staining. Upon encountering fixative, conjugated antibodies can potentially lose some of their signal. The impact of fixative on SuperNova v428 conjugated antibodies after staining, as well as stained and fixed whole blood sample stability, were assessed using the IOTest3 Lysing Solution protocol and a CD22-SNv428 conjugated antibody, with the same normal whole blood (100μL: 5x105 cells/test) sample being acquired on the flow cytometer either directly after staining (no fixation) or 24 hours post-staining after formaldehyde fixation. The same sample was stained and acquired in the same conditions with Pacific Blue and BV421 CD22 conjugates for comparison.
Figure 9 shows the lymphocyte RMFI obtained for each condition tested.
Figure 9. Comparison of a CD22-SuperNova v428, Pacific Blue and BV421 staining on a normal whole blood sample with IOTest3 Lysing Solution, acquired without formaldehyde fixation directly and with formaldehyde fixation at 24h, showing RMFI values (top) and flow cytometry staining data (bottom).
Figure 9 shows that the CD22-SNv428 is not sensitive to formaldehyde-based fixation, and once fixed, the stained sample can be acquired 24 hours later, similarly as with Pacific Blue and BV421. The CD22-SuperNova v428 staining index was slightly better 24h post-acquisition, improvements probably due to the formaldehyde fixation.
Tips and tricks for a successful experiment:
- SuperNova v428 is not sensitive to formaldehyde-based fixatives.
- SuperNova v428 is compatible with low- and high-fixation protocols from the PerFix-nc intracellular kit.
- A fixed sample stained with CD22-SuperNova v428 is stable at least 24 hours prior to acquisition, providing greater flexibility in experiments.
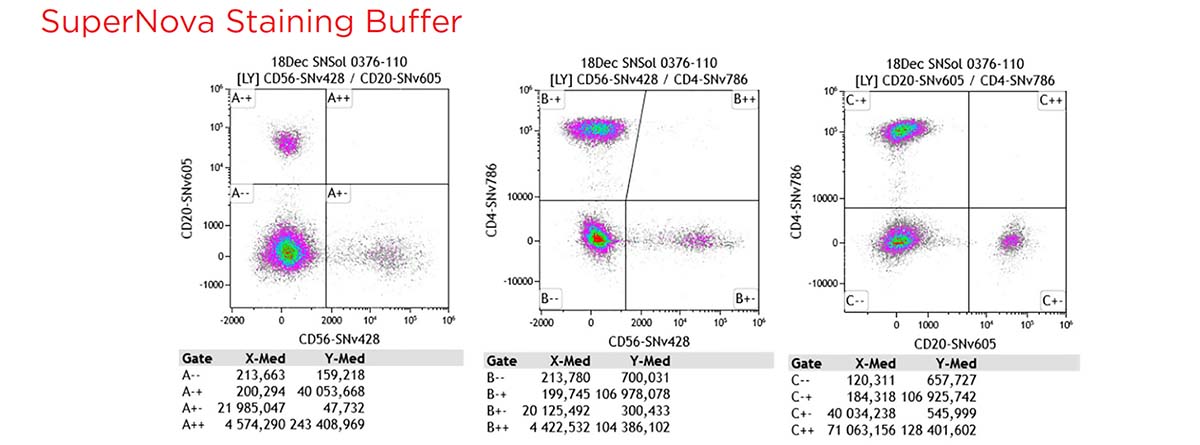
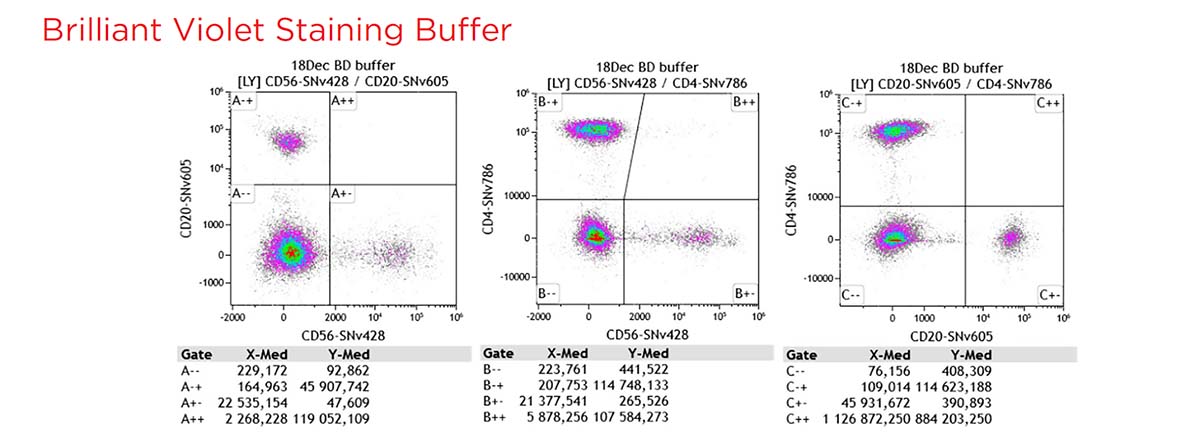
To assess the compatibility of the SuperNova v428 conjugates in multicolor flow cytometry experiments, a 12 colors cocktail with 3 SuperNova violet conjugates + 9 regular conjugates have been tested, on normal whole blood (100 μL: 5x105 cells/test), with and without the SuperNova and the Brilliant Violet staining buffer.
12 colors panel: CD16-FITC, CD197-PE, CD45RO-ECD, TCR PAN g/d-PC5.5, CD45 RA-PC7, CD19-APC, CD127-APC-Alexa Fluor 700, CD3-APC-Alexa Fluor 750, CD56-SNv428, CD8-KrO, CD20-SNv605, CD4-SNv786.
Protocol:
- Add 10 μL of SuperNova staining buffer or 10 μL of Brilliant Violet buffer or nothing (« no buffer »).
- Add SuperNova Conjugates first, and then the other conjugates, vortex.
- Add 100 μL of whole blood, vortex, incubation 15-20 min at room temperature.
- Lyse, fix and wash the sample using VersaLyse protocol.
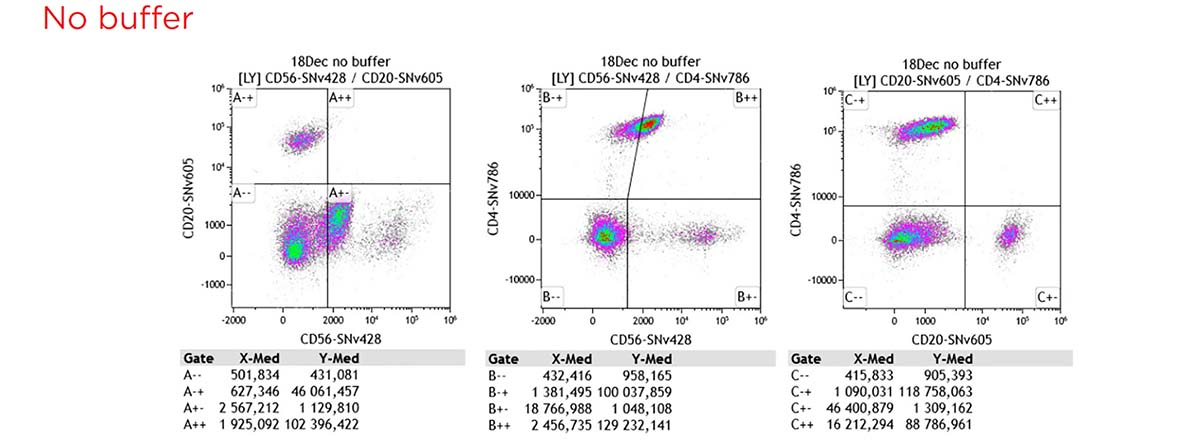
Figure 10 shows the gating strategy and staining patterns.
Without buffer interactions across polymer dyes are observed. This phenomenon is well-known with other commonly used polymer dyes and also observed with SuperNova dyes. A specific staining buffer is recommended.
The compatibility of SuperNova v428 with traditional fluorochromes has been demonstrated.
The SuperNova and the Brilliant Violet staining buffers reduce significantly the interactions across polymer dyes.
Figure 10. Gating strategy and staining pattern for the 12 colors cocktail, without additional buffer, with SuperNova staining buffer and with BV staining buffer.
Table 3 shows compensation matrix and the channel gain table for the 12 Colors panel:
Table 3. Channel gain table and compensation matrix for the 12 colors panel.
| FL1 | FL2 | FL3 | FL4 | FL5 | FL6 | FL7 | FL8 | FL9 | FL10 | FL11 | FL13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FL1 | 0,50 | 0,97 | 0,05 | 0,06 | 0,00 | 0,19 | 0,03 | 0,17 | 1,30 | 0,00 | 0,09 | |
| FL2 | 25,54 | 15,35 | 1,52 | 1,55 | 0,00 | 0,12 | 0,02 | 0,23 | 1,17 | 1,66 | 0,05 | |
| FL3 | 10,34 | 46,50 | 0,75 | 0,74 | 0,00 | 0,03 | 0,01 | 0,21 | 0,88 | 11,63 | 0,04 | |
| FL4 | 3,35 | 13,92 | 47,97 | 0,71 | 0,86 | 1,26 | 0,08 | 0,22 | 0,68 | 11,30 | 0,17 | |
| FL5 | 0,45 | 2,55 | 11,05 | 53,92 | 0,12 | 1,30 | 1,80 | 0,06 | 0,51 | 4,07 | 14,46 | |
| FL6 | 0,30 | 0,00 | 1,31 | 1,40 | 0,03 | 6,21 | 5,35 | 0,09 | 0,02 | 2,00 | 0,39 | |
| FL7 | 0,44 | 0,00 | 0,89 | 25,76 | 0,05 | 31,30 | 2,32 | 0,13 | 0,14 | 1,47 | 0,94 | |
| FL8 | 0,06 | 0,00 | 0,52 | 11,41 | 5,70 | 13,20 | 48,11 | 0,11 | 0,22 | 0,78 | 30,23 | |
| FL9 | 1,46 | 0,00 | 0,53 | 0,04 | 0,00 | 0,00 | 0,00 | 0,01 | 6,29 | 15,64 | 7,58 | |
| FL10 | 2,47 | 0,00 | 0,28 | 0,04 | 0,00 | 0,00 | 0,00 | 0,00 | 4,70 | 0,71 | 0,97 | |
| FL11 | 1,01 | 2,36 | 5,27 | 0,03 | 0,03 | 0,00 | 0,00 | 0,02 | 0,49 | 63,09 | 0,36 | |
| FL13 | 0,11 | 0,04 | 0,80 | 1,56 | 2,74 | 0,19 | 1,24 | 2,35 | 0,00 | 3,61 | 32,48 |
Compensation requirements may vary depending on the instrumentation setting and application, each lab should define its own compensation requirements.
| Channel | FL1 | FL2 | FL3 | FL4 | FL5 | FL6 | FL7 | FL8 | FL9 | FL10 | FL11 | FL13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conjugate | CD16-FITC | CD197-PE | CD45RO-ECD | TCR PAN γ/δ-PC5.5 | CD45RA-PC7 | CD19-APC | CD127-A700 | CD3-APC-A750 | CD56-SNv428 | CD8-KrO | CD20-BV605 | CD4-BV786 |
| Gain | 92 | 82 | 154 | 199 | 490 | 1081 | 961 | 822 | 83 | 34 | 100 | 200 |
Tips and tricks for a successful experiment:
- SuperNova v428 is compatible with most traditional fluorochromes.
- The SuperNova staining buffer is recommended when multiple polymer dyes are used in a panel, to prevent interactions across polymer dyes. Alternatively, the BV staining buffer can also be used.
- Out of the violet laser, use SuperNova v428 conjugates for dimly expressed markers, Pacific Blue and Krome Orange are optimal for gating or strongly expressed markers.
For the staining of fragile cells, such as PBMC preparations or samples that went through washing steps prior to staining, resuspension in proteins such as FCS (Fetal Calf Serum) might help ensuring cell integrity.
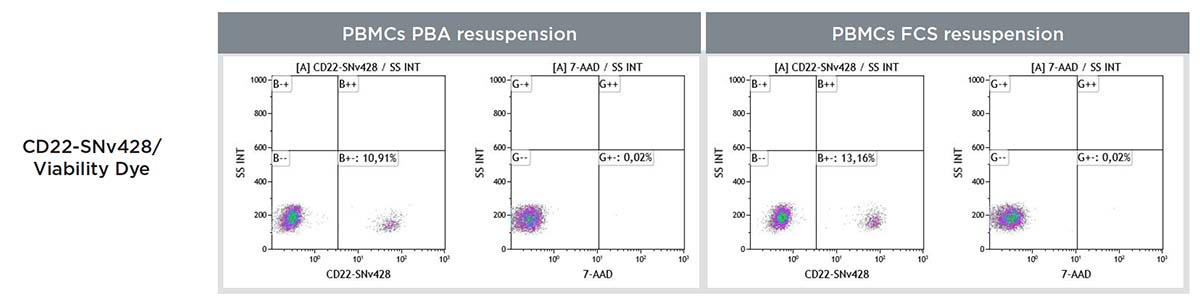
Figure 11 shows staining of a CD22-SNv428 on PBMC in a FCS and a PBS 1X BSA 2mg/mL NaN3 0.1% (PBA) resuspension.
Figure 11. CD22-SNv428 staining on PBMC including cell viability with the 7-AAD staining, two PBMC resuspension protocols using PBS 1X BSA 2 mg/mL or FCS 100% have been compared. The CD22-SNv428 and 7-ADD signals are observed in a single versus a dual polymer dye conjugates staining context.
The use of FCS in the resuspension ensures cell viability is not impacted.
The following protocol was defined as optimal for staining PBMCs (CD22-SNv428 used as an example):
PBMC preparation:
- Put 4 mL of Heparin whole blood + 4 mL PBS 1X in a 15 mL tube
- In another 15 mL tube, put 3 mL of Ficoll Solution and add very slowly 4 mL of the mixed whole blood/PBS1X
- Centrifuge 30 min at 400 x g without deceleration
- Retrieve the PBMC “ring” in a 15 mL falcon tube
- Add 9 mL of PBS 1X FCS 10%
- Centrifuge 10 min at 400 x g with deceleration
- PBMC are prepared using a classical Ficoll solution but with a final resuspension in 500 μL FCS 100%
PBMC staining:
- In a cytometric tube, add 100 μL (5.105 cells/test) of prepared PBMC in FCS 100%
- Add 10 μL of polymer dye conjugate (for this experiment 10 μL of CD22-SNv428) and 10 μL of 7-ADD reagent were included to assess cell viability.
- Incubate for 20 min
- Add 3 mL of PBS 1X Buffer
- Centrifuge 5 min at 300 x g
- Resuspend with 500 μL FCS 100%
Results
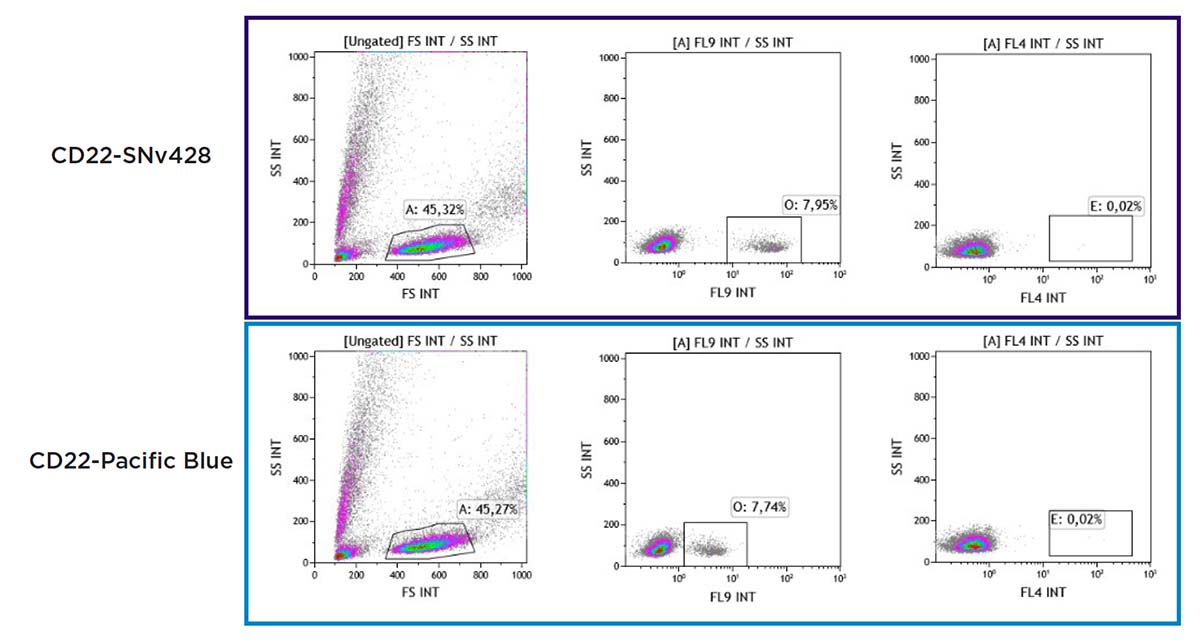
As shown in Figure 12, a similar percentage of CD22+ B cells is gated with CD22-SuperNova v428 and CD22-Pacific Blue. The SuperNova v428 conjugate leads to significantly brighter staining. The use of FCS in the resuspension ensures cell viability is not impacted.
Figure 12. PBMC staining comparison with CD22 conjugated to SuperNova v428 and Pacific Blue, including cell viability.
Tips and tricks for a successful experiment:
- PBMC are more fragile compared to whole blood, as they are not protected by plasma elements, and cell integrity can be affected by the conjugated antibody formulation.
- Resuspending in 100% Fetal Calf Serum (FCS) protects the cells and preserves cell viability.
- This FCS addition isn’t an obligation for a single polymer dye staining but is recommended for a multi polymer dye staining on PBMC.
- This trick can be used for other protocols with fragile cells or involving washing steps prior to staining (e.g., bulk lysis – wash – stain protocol).
For certain applications, whole blood needs to be washed before staining, and resuspension in protein is recommended to ensure cell integrity.
After wash blood steps we tested several cell resuspension buffers containing:
- PBS1X
- PBS1X BSA 2mg/mL NaN3 0,1% (PBA)
- PBS1X FCS 2%
- PBS1X FCS 10%
- FCS 100%
In comparison with a no-wash whole blood protocol, after resuspension a classic staining with VersaLyse Wash/Fixation was done.
Tests were done in duplicate, and as no difference in results were observed, an average was done for each.
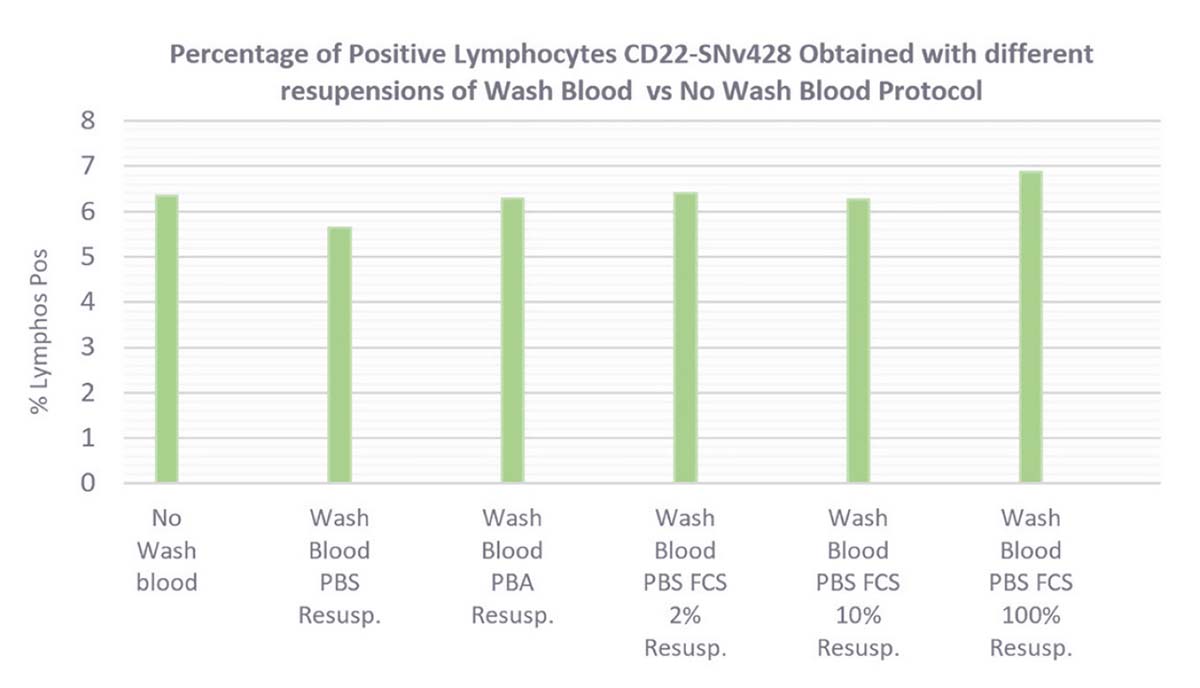
Figure 13 in green shows the percentage of positive lymphocytes. It hasn’t been impacted by the blood washing step. The percentage of positive lymphocytes is equivalent between wash and no-wash whole blood protocols.
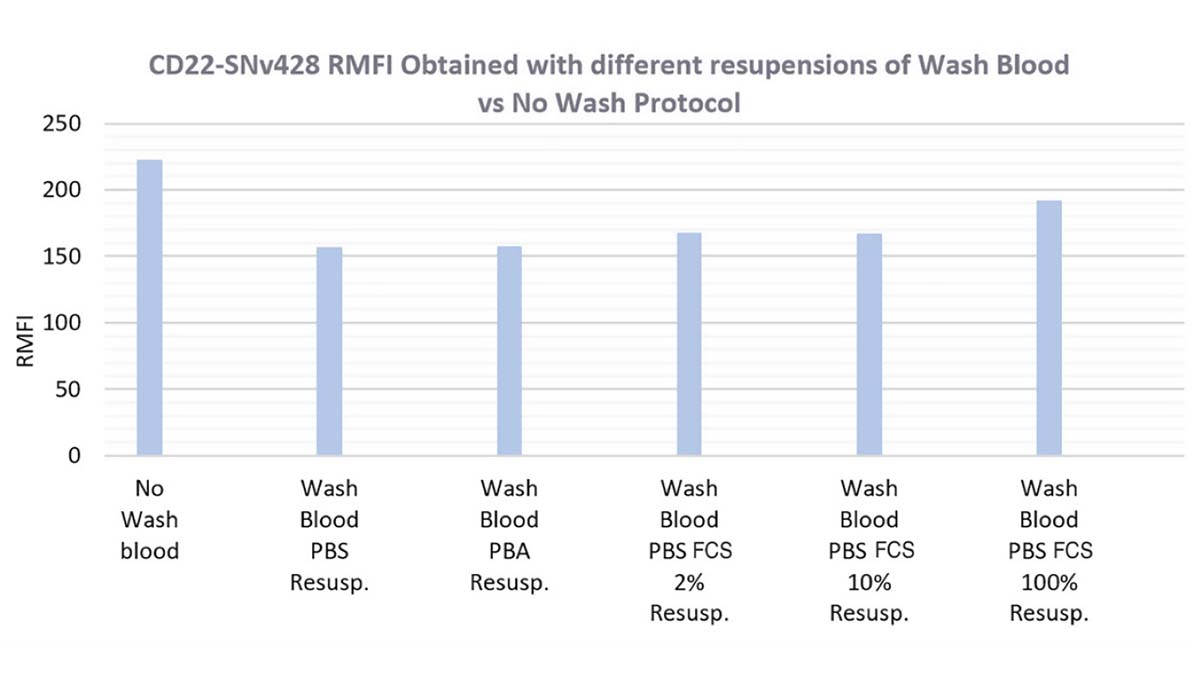
In Blue the CD22-SNv428 RMFI is impacted, the PBS 1X resuspension (without protein) shows a low RMFI in comparison to the no-wash whole blood CD22 RMFI. BSA at 2 mg/mL, 2% or 10% FCS in PBS1X does not improve RMFI.
Only cell resuspension in SVF 100% increases the CD22-SNv428 RMFI at 86% of the RMFI obtained with no wash whole blood procedure.
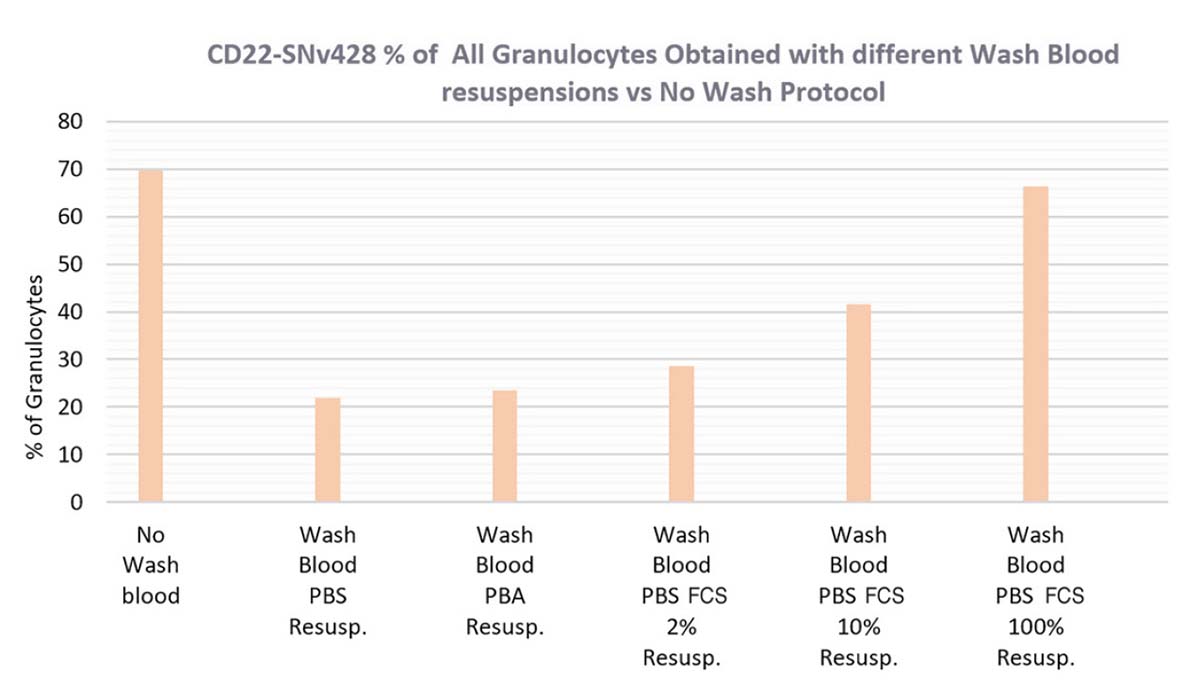
In orange, the granulocytes percentage is impacted in all wash whole blood protocols. Adding proteins resolves this issue, but only a cell resuspension in SVF 100% demonstrates an equivalent percentage of granulocytes to the nowash whole blood procedure.
Figure 13. CD22-SNv428 Percentage of Positive Lymphocytes (in green), RMFI (in blue) and Percentage of Negative Granulocytes (in orange), obtained with different resuspension cells after Wash Blood steps (PBS1X, PBA, PBS1X FCS2% and 10%, FCS100%) versus No-Wash Whole Blood Protocol.
Tips and tricks for a successful experiment:
- Washed whole blood cells are more fragile, it is therefore recommended to resuspend in 100% Fetal Calf Serum (FCS) to protect and preserve the cells.
- This trick can be used for other protocols with fragile cells.
Conclusion
SuperNova polymer dyes are compatible with most protocols commonly used in flow cytometry labs. This next generation of polymer dyes are brighter than other conventional and polymer dyes excited by the violet laser and generate significantly less non-specific staining thanks to a proprietary formulation, proving greater confidence in results. Therefore, SuperNova dyes conjugated antibodies are optimal for the assessment of dimly expressed cell populations.
SuperNova v428 can easily be integrated into existing panels using a 450/50 bandpass filter or equivalent. Spillover is similar to BV421, leading to ~5-10% compensation required in the FL10 channel. When multiple polymer dyes are used in the same panel, the SuperNova staining buffer is recommended.
SuperNova v428 conjugated antibodies are compatible with commonly used Beckman Coulter lysing solution and also with formaldehyde-based fixation. A washing step is recommended to obtain optimal staining index. For staining of PBMCs or other fragile cells, resuspension in an FCS or equivalent buffer is recommended to preserve cell integrity. Resuspension in FCS is also recommended if the sample is lysed-washed prior to staining.
In addition to the optimization of individual conjugated antibody performance, the associated manufacturing processes have been optimized and validated to meet clinical and current Good Manufacturing Practices (cGMP) requirements for catalog products and deliver conjugates with low variability lot after lot.
For Research Use Only - Not for use in diagnostic procedures.